Actinium Pharmaceuticals Presents New Preclinical Data Demonstrating Potent Anti-Tumor Activity of ATNM-400 Across Multiple Breast Cancer Subtypes Including Hormone Receptor-Positive, Triple-Negative, and Tamoxifen- and HER2 Therapy-Resistant Breast Cancer Models at SABCS 2025
- ATNM-400's target antigen is overexpressed in breast cancer and its expression is further increased in breast cancer cells resistant to standard of care endocrine therapy, tamoxifen and the HER2 therapy trastuzumab
- The Actinium-225 alpha-emitter payload of ATNM-400 induced irreversible double-strand DNA breaks and has the potential to produce potent localized tumor killing with reduced off-target lung toxicity that limits the use of antibody drug conjugates
- Data supports the continued development of ATNM-400 as a monotherapy or combination therapy to address multiple breast cancer subtypes with limited treatment options
Highlights from the SABCS 2025 Poster Presentation
The poster, titled "Anti-Tumor Activity of ATNM-400, a First-in-Class Actinium-225 Antibody Radioconjugate, in Hormone-Positive, Triple-Negative, Tamoxifen-Resistant and Trastuzumab-Resistant Breast Cancer Models," showcases the following key findings:
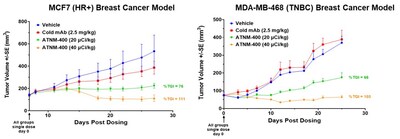
Potent Efficacy Across Breast Cancer Subtypes: ATNM-400 demonstrated significant tumor-growth inhibition (TGI) in HR+ (MCF7) and TNBC (MDA-MB-468) in vivo models, with all treatment regimens well tolerated and no significant changes in body weight observed.
Potent Activity in Standard-of-care (SOC) Treatment-Resistant Breast Cancer Models:
- Trastuzumab-resistant BT474-Clone5 breast cancer cells or Tamoxifen-resistant MCF7-Tam1 breast cancer cells exhibited increased target expression, resulting in enhanced in vitro cytotoxicity with ATNM-400.
- Combining ATNM-400 with either trastuzumab or tamoxifen resulted in greater cytotoxicity versus monotherapy and produced in vivo tumor regression in the trastuzumab-resistant model.
Mechanistic Evidence of Irreversible DNA Damage
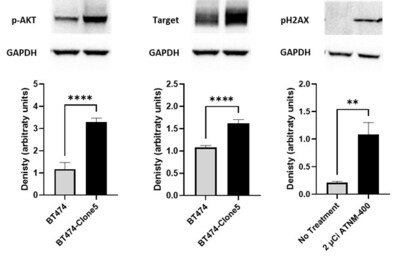
- Activation of phosphorylation of AKT (pAKT) was observed in trastuzumab resistant BT474-Clone5 breast cancer cells, as well as a significant increase in the total level of the ATNM-400 target antigen in the in vivo trastuzumab-resistant breast cancer model.
- ATNM-400 treatment of these trastuzumab-resistant breast cancer cells caused significant increase in phosphorylated H2AZ (pH2AX), consistent with alpha-particle–driven double-strand DNA damage.
Favorable Biodistribution: Sustained tumor uptake in a breast cancer model through 144 hours and rapid clearance from normal organs supports a potentially differentiated safety profile.
Pan-Tumor Potential: These results, together with previously published ATNM-400 data in prostate and lung cancer, reinforce the program's broad applicability across solid tumors.
Dr.
"Patients whose tumors progress after endocrine therapy or HER2-targeted therapies face limited and often toxic treatment options. The data presented at SABCS show that ATNM-400 generates potent, targeted DNA damage even in highly resistant breast cancer models, while maintaining a favorable tolerability profile. This therapeutic approach has the potential to address some of the most pressing unmet needs in breast cancer care, particularly for those who have exhausted established modalities", said
Breast cancer remains a heterogeneous disease with significant unmet need, particularly for patients who relapse following endocrine or HER2-directed therapies—an issue impacting 20–30% of HR+ patients and many with HER2-positive disease. In this setting, tamoxifen and trastuzumab (Herceptin®, Roche and biosimilars) generated sales of approximately
The ATNM-400 SABCS poster presentation can be access on Actinium's investor relations website HERE.
About ATNM-400
ATNM-400 is a highly innovative, first-in-class, and multi-indication Actinium-225 (Ac-225) targeted radiotherapy candidate in development for prostate cancer, non-small cell lung cancer (NSCLC) and breast cancer. ATNM-400 is highly differentiated in prostate cancer as it targets a distinct non-PSMA protein strongly implicated in prostate cancer disease biology including progression and treatment resistance. Unlike 177Lu-PSMA-617, the active agent in Pluvicto® and the majority of radiotherapies under development, which rely on PSMA targeting, ATNM-400 is designed to maintain efficacy in low-PSMA or high-PSMA resistant disease, a major unmet clinical need as up to 30% of patients do not respond to PSMA radioligand therapies and up to 60% of patients have at least one PSMA-negative tumor lesion. Ac-225 delivers high-linear-energy-transfer alpha particles that induce irreparable double-strand DNA breaks, offering superior potency over beta emitters like Lutetium-177 (177Lu), and has a shorter tissue path length that may reduce off-target toxicity. The receptor specifically targeted by ATNM-400 continues to be expressed at a high level even after androgen receptor inhibitor (ARPI) and ATNM-400 has shown to overcome resistance to the ARPI therapy enzalutamide and work synergistically in combination with enhanced tumor control including complete tumor regression. In NSCLC, ATNM-400 has shown superior efficacy compared to approved first, second- and third-line EGFR therapies including small molecules, antibody drug conjugates and bispecific antibodies that is synergistic with osimertinib, an EGFR tyrosine kinase inhibitor (TKI) that is a standard of care therapy approved for treatment of patients in the frontline setting and is also able to overcome osimertinib resistance. ATNM-400 has also shown potent anti-tumor activity across multiple breast cancer subtypes including hormone receptor-positive, triple-negative, and tamoxifen- and HER2 therapy-resistant breast cancer models.
Prostate cancer is the most commonly diagnosed cancer in men, with ~1.5 million new cases globally and over 313,000 expected in the
About
Actinium is a pioneer in the development of targeted radiotherapies intended to meaningfully improve patient outcomes. ATNM-400, Actinium's lead product candidate, is a novel, first-in-class, and multi-indication Actinium-225 (Ac-225) in development for prostate cancer, non-small cell lung cancer (NSCLC) and breast cancer. The antigen specifically targeted by ATNM-400 is highly expressed in metastatic castration-resistant prostate cancer (mCRPC), contributes directly to disease progression, poorer survival outcomes, and continues to be expressed at a high level even after androgen receptor inhibitor (ARPI) and Pluvicto® treatment. ATNM-400 is supported by preclinical data demonstrating tumor-specific uptake, higher efficacy than androgen receptor inhibitor enzalutamide (Xtandi®) and 177Lu-PSMA-617 radiotherapy, the active agent in Pluvicto®, durable tumor control and potent efficacy in prostate cancer models resistant to both enzalutamide and 177Lu-PSMA-617. In addition, ATNM-400 has demonstrated synergy with enzalutamide. In NSCLC, ATNM-400 showed superior efficacy to EGFR targeting therapies including osimertinib (TARGRISSO®, AstraZeneca), Dato-DXd (DATROWAY®, AstraZeneca/Daiichi Sankyo) and amivantamab (RYBREVANT®, J&J) with synergistic activity in combination with osimertinib. In breast cancer, ATNM-400 showed potent anti-tumor activity across multiple breast cancer subtypes including hormone receptor-positive, triple-negative, and tamoxifen- and HER2 therapy-resistant breast cancer models. The data generated to date with ATNM-400 supports its potential across treatment settings to be used either as a monotherapy, or in combination or sequenced with other therapies. Actinium's most advanced product candidate in development is Actimab-A, a CD33 targeting therapeutic, that is a potential backbone therapy for acute myeloid leukemia (AML) and other myeloid malignancies leveraging the mutation agnostic alpha-emitter radioisotope payload Actinium-225 (Ac-225). Actimab-A has demonstrated potential activity in relapsed and refractory acute myeloid leukemia (r/r AML) patients in combination with the chemotherapy CLAG-M including high rates of Complete Remissions (CR) and measurable residual disease (MRD) negativity leading to improved survival outcomes and is being advanced to a pivotal Phase 2/3 trial. In addition, Actinium is engaged with the
For more information, please visit: https://www.actiniumpharma.com/
Forward-Looking Statements
This press release may contain projections or other "forward-looking statements" within the meaning of the "safe-harbor" provisions of the private securities litigation reform act of 1995 regarding future events or the future financial performance of the Company which the Company undertakes no obligation to update. These statements are based on management's current expectations and are subject to risks and uncertainties that may cause actual results to differ materially from the anticipated or estimated future results, including the risks and uncertainties associated with preliminary study results varying from final results, estimates of potential markets for drugs under development, clinical trials, actions by the FDA and other governmental agencies, regulatory clearances, responses to regulatory matters, the market demand for and acceptance of Actinium's products and services, performance of clinical research organizations and other risks detailed from time to time in Actinium's filings with the Securities and Exchange Commission (the "
Investors:
investorrelations@actiniumpharma.com
![]() View original content to download multimedia:https://www.prnewswire.com/news-releases/actinium-pharmaceuticals-presents-new-preclinical-data-demonstrating-potent-anti-tumor-activity-of-atnm-400-across-multiple-breast-cancer-subtypes-including-hormone-receptor-positive-triple-negative-and-tamoxifen--and-her2-thera-302640194.html
View original content to download multimedia:https://www.prnewswire.com/news-releases/actinium-pharmaceuticals-presents-new-preclinical-data-demonstrating-potent-anti-tumor-activity-of-atnm-400-across-multiple-breast-cancer-subtypes-including-hormone-receptor-positive-triple-negative-and-tamoxifen--and-her2-thera-302640194.html
SOURCE